Use of Pneuma in patients with COVID-19
The PNEUMA development process focused on the identification of the requirements for a low-cost ventilator that could resort to resources available (e.g. self-inflating bag), based on the needs of several healthcare professionals.
Manual ventilation with a self-inflating bag is an alternate solution in a critical care setting, with no clinical evidence supporting its long-term use (i.e. days). This is not a ventilation method associated with a pulmonary protection strategy, but rather an alternative.
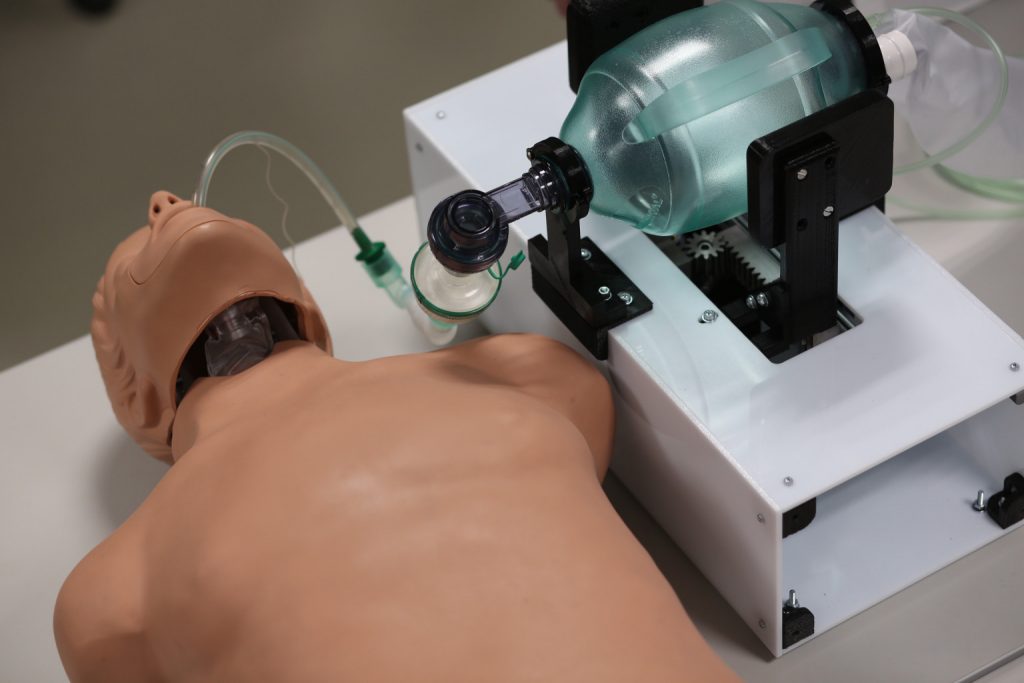
Any solution adopted in a clinical setting should only be used under the supervision of a healthcare professional. According to the current European and national regulations, and in our understanding, PNEUMA is considered a class I device, subject to EC self-certification.
In the current context, PNEUMA does not hold said certification. The device shall not replace any properly certified hospital ventilator. However, in the setting of the COVID-19 pandemic, it can be quite useful to relieve existing ventilators or in other life threatening situations.